Lysoptosis – A lysosome-mediated cell death
For details, see An intracellular serpin regulates necrosis by inhibiting the induction and sequelae of lysosomal injury.
The majority of human diseases result in drastically altered cellular homeostasis. If the cell cannot compensate for these changes, cellular dysfunction and damage occurs, resulting in cell death and disease progression. Promiscuous proteolytic activity from protease enzymes can be the major executioners of multiple cell death pathways, which are blocked, in part, by endogenous inhibitors such as serpins. We focus on the relationship between cell stress (e.g., infection, hypoxia, hyperoxia, and electrolyte disturbances), protease activation, and the serpin blockade. Using multiple technologies (e.g., live-cell imaging, chemical mutagenesis, RNAi, and CRISPR/Cas9) in C. elegans, mouse, and cell culture models of human diseases, we show that intracellular serpins serve as pro-survival factors. Certain serpins neutralize lysosomal cysteine proteases, and block a novel form of regulated cell death, lysoptosis. This cell death pathway is activated, particularly in epithelial cells, after major cellular insults, as occurs in multiple diseases of newborns and adults. Using state-of-the-art technologies, we are further delineating the genetic and molecular basis of this pathway and compounds that can modulate this pathway.
For details, see Lysoptosis is an evolutionarily conserved cell death pathway moderated by intracellular serpins.
Serpinopathies and other conformational disorders
Serpins are metastable proteins and mutations in serpin genes can lead to protein misfolding, cellular stress and injury. The canonical serpinopathy, a1-antitrypsin (AT) deficiency (ATD), results in the polymerization/aggregation and retention of AT in the ER of liver cells where it causes inflammation, cirrhosis, and hepatocellular carcinoma. Currently, organ transplantation is the only fully effective treatment for ATD liver disease. To develop new therapeutic strategies, we developed a model of ATD in C. elegans that recapitulates the important cellular aspects of the human disease. We have also developed a live-animal, semi-automated high-throughput platform for conducting small molecule and genetic screens for identifying hit compounds and modifier genes that serve as new therapeutic targets, respectively. We have also developed a tauopathy model in C. elegans and using the same high-throughput strategies to identify new drugs to treat the disease.
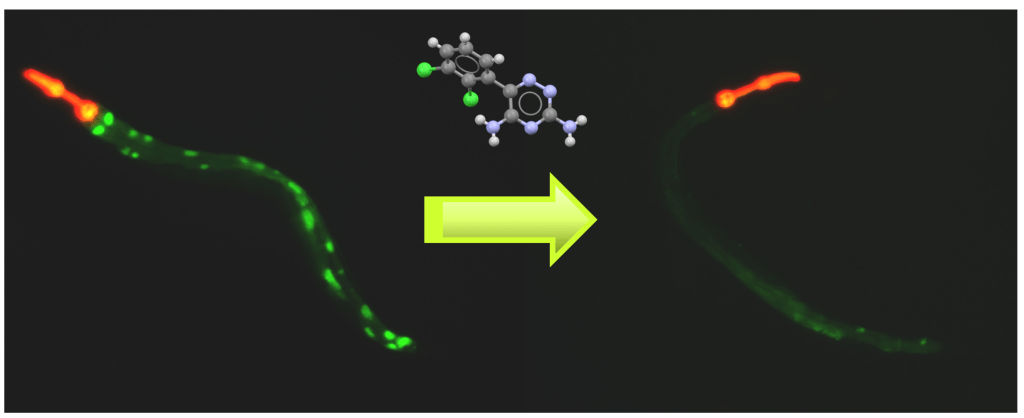
Autophagy and disease
Autophagy is a fundamental cellular process that plays a critical role in the clearance of damaged or unwanted nucleic acids, proteins and organelles and is required for normal cellular homeostasis. Autophagy dysfunction contributes to the pathogenesis of a broad range of systemic disorders including diabetes, myopathies, premature ageing, cancer, hepatic failure, interstitial lung disease, pancreatitis, autoimmunity, and neurodegeneration. EPG5 is an important protein that facilitates the fusion of autophagosomes with lysosomes. Genetic mutations in the EPG5 gene can cause Vici Syndrome, a devastating disease that affects multiple systems including the brain, heart, and the muscles. We have developed a model of Vici Syndrome in C. elegans to identify genetic suppressors to develop therapeutic strategies for reversing the effects of EPG-5/autophagy dysfunction.
Undiagnosed diseases
Rare diseases affect more than 300 million people worldwide. In spite of the advances in whole genome sequencing and bioinformatic tools, over 80% of these cases remain undiagnosed. As part of the NIH’s Undiagnosed Diseases Network (UDN), we use C. elegans to rapidly assess the pathogenicity of variants of uncertain significance (VUS). Our modeling has three goals: 1) determine if the variant is damaging to gene function, 2) understand the genetic mode of inheritance (loss-of-function or gain-of-function (dominant-negative, hyperactive or neomorphic)), and, where possible, 3) investigate the mechanism of disease. These functional data provide support for identification of new disease genes, and help provide a diagnosis for the patients and their families.
For further information…